Many types of light microscopes have been discovered and they are called as
per their light background as well as their functioning. They have different
properties also (Table 1). The light microscopes are of following types:
1. Bright-field microscope
2. Dark –field microscope
3. Phase-contrast microscope
4. Fluorescence microscopes
These are compound microscopes as image is formed by action of two or more
than lenses.
1. The Bright –Field Microscope
This microscope produces a dark image against a brighter background. There
are several objective lenses are present in the microscope.
Total magnification is the product of the magnifications of the ocular lens and
the objective lens.
Microscope Resolution is the ability of a lens to separate or distinguish small
objects that are close together. Wavelength of light used is a major factor in the
resolution. As we have discussed earlier that if we use shorter wavelength, there
will be greater resolution.
1. The Dark-Field Microscope
This microscope produces a bright image of the object against a dark
background. It is used to observe living, unstained preparations.
1. The Phase-Contrast microscope
It enhances the contrast between intracellular structures having slight
differences in refractive index. This microscope is excellently used to observe
living cells.
Working of Phase –Contrast Microscope:
In microscopy there is a small phase shifts in the light passing through a
transparent specimen are converted into amplitude or contrast changes in the
image. A phase contrast microscope does not require staining to view the object
as it is used to study living cells. This microscope made it possible to study the
cell cycle very comfortably.
As light travels through a medium other than vacuum, it causes its amplitude
and phase to change in a way which depends on properties of the medium. This
change in amplitude give rise to familiar absorption of light which gives rise to
colours as it is wavelength dependent. Our eye measures only the energy of
light arriving on the retina, so changes in phase are not easily observed, yet
often these changes in phase carry a large amount of information.
To make phase variations observable, it is necessary to combine the light
passing through the sample with a reference so that the resulting interference
reveals the phase structure of the sample; same is done using Phase-contrast
microscope. Frits Zernike is given credit of discovering the Phase-Contrast
Microscope. He was awarded the Nobel Prize (physics) in 1953.
In this microscopy, the necessary phase change is introduced by rings etched
accurately onto glass plates so that they introduce the required phase change
when inserted into the optical path of the microscope during study. This
technique allows phase of the light passing through the object under study to be
inferred from the intensity of the image produced by the microscope.
A phase ring is responsible cover the phase change, which is located in a
conjugated aperture plane somewhere behind the front lens element of the
objective and a matching annular ring, which is located in the primary aperture
plane; this is the location of the condenser's aperture.
Two selected light rays, which are emitted from the light source, get focused by
the lens inside the opening of the condenser annular ring. These two light rays
are then refracted in such way that they exit the condenser lens as parallel rays.
We may assume that the two rays in question are neither refracted nor
diffracted in the specimen plane, they enter the objective as parallel rays. Since
all parallel rays are focused in the back focal plane of the objective, the back
focal plane is a conjugated aperture plane to the condenser's front focal plane;
this is the location of the condenser annulus. To complete the phase setup, a
phase plate is positioned inside the back focal plane in such a way that it lines
up nicely with the condenser annulus.
You must know that phase-shift of 90° (λ/4) due to objects are balanced
again90° (λ/4) by phase plates. The recombination of these two waves in the
primary image plane results in a significant amplitude change at all locations
where there is a now destructive interference due to a 180° (λ/2) phase shift.
The net phase shift of 180° (λ/2) results directly from the 90° (λ/4) retardation
of the phase object and the 90° (λ/4) phase advancement of the wave due to the
phase plate.
The Differential interference Contrast Microscope creates image by detecting
differences in refractive indices and thickness of different parts of specimen.
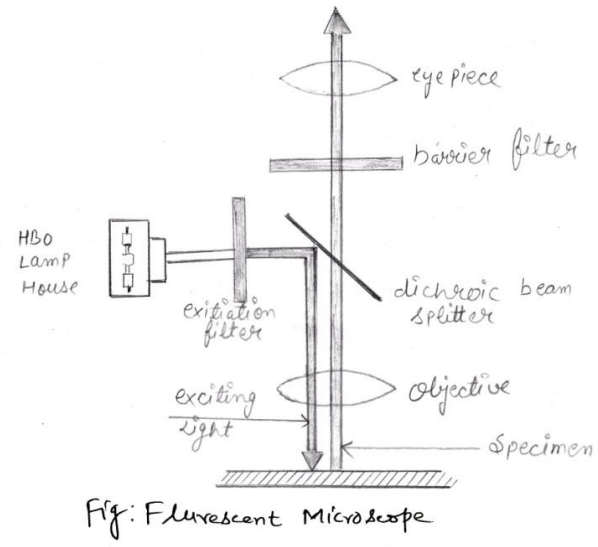
2. The Fluorescence Microscope
This microscopy exposes specimen to ultraviolet violet, or blue light.
Specimens usually stained with fluorochromes, which emits fluorescent light while exposed against light. It results in a bright image of the object resulting
from the fluorescent light emitted by the specimen.
Working of the Fluorescence Microscope
Barrier filter are there in this microscopy which removes any remaining exciter
wavelengths (up to about 500mm) without absorbing longer wavelengths of
fluorescing object. As told already that specimen stained with fluorochrome
emits fluorescence when activated by wavelength of light; especially darl-field
condenser provides dark background for fluorescence.
3. Polarized Light Microscopy
Basic principles of polarized light
You must have read in 10+2 physics class that ordinary light contains light
waves that vibrate in a direction perpendicular to its direction of travel.
Especially, Polarized light is used only as a means of rendering microstructures
visible in non-cubic metals or polymers. For generating polarized light,
ordinary light must pass or be reflected by a polarizing device. This device will
absorb all directions of vibration except the permitted direction. This light
emerging from the interaction called polarized light. Polarized light not only
elucidates identifying parameters, it often detects delicate changes also.
Polarized light is known for two distinct phenomenons: 1. the nature of the
incoming light and 2. the internal characteristic of the material. Polarized light
enhances contrast based on the difference in refractive indices in at least two directions in a material used. For example, a drawn fiber will have two
refractive indices: first along its length and second across its diameter.
In polarized microscope, amorphous and crystalline regions in a polymer will
respond to polarized light through interference. If we use the dark field setting
on the cross polarizer, the amorphous part of the polymer is optically
transparent and will appear tan in the image while the light passing through the
crystalline regions will appear white. It occurs because the crystals lie along the
transmission axis of the light.
Again during the study of light field measurements, the crystalline regions will
react with destructive interference with the light while the amorphous regions
will react as before with the light. These two phenomena are responsible for
image formation in Polarized Light Microscopy.
4. UV Light Microscopy
Application of light beam with a shorter wavelength using UV light is also
possible resulting in higher resolving power. Using UV light the resolution can
be reduced to 0.1 μm, but special quartz lenses and UV-light detector are
essential, so that the light microscope with UV light source is only a theoretical
possibility.
7. Classical Interference Microscopy
In this microscopy there is use of two separate light beams with much greater
lateral separation than that used in phase contrast microscopy.
Due to use of two beams the interference microscope is having special features
where object and reference beam pass through the same objective, two images
are produced of every object (one being the "ghost image"). These two images
are separated either laterally within the visual field or at different focal planes.
These two images can be a overlapping sometimes, since they can severely
affect the accuracy of mass thickness measurements. Rotation of the
preparation is used to avoid this problem.
The main advantage of interference microscopy measurements is to explore
measuring the projected dry mass of living cells, which was first effectively
exploited by Andrew Huxley in studies of striated muscle cell structure and
function, leading to the sliding filament model of muscle contraction.






0 Comments